Design and Rationale
Despite major advances in our understanding of genetic cardiomyopathies, they remain the leading cause of premature sudden cardiac death and end-stage heart failure under the age of 60 years. To provide a scaffold for future research, we developed the UNRAVEL RDP.
Using routine electronic health records and standardized biobanking, big data analysis on a larger number of patients and investigations are made possible. The UNRAVEL research data platform is embedded in routine practice to facilitate research in genetic cardiomyopathies. The paper has been published in the Netherlands Heart Journaland can be downloaded here.
Eligible Participants
Eligible participants are patients with proven or suspected cardiac disease and their relatives are asked for permission to use their data and draw blood for biobanking. Routinely collected clinical data is included in a research database by weekly extraction.
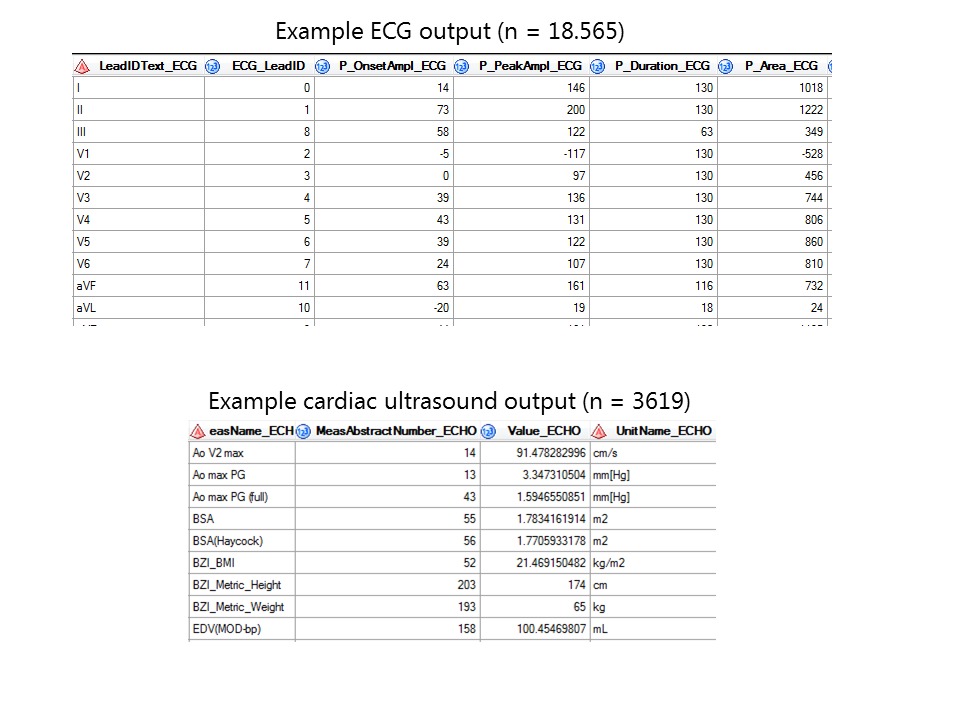
Thus far, 1000 individuals have been included with a median age of 57, of which 57% are men. All data is captured in a temporal sequence amounting to a total of 18,565 electrocardiograms, 3619 cardiac ultrasounds, 20,000 radiological examinations and 650,000 individual lab measurements.
Ethics
The UNRAVEL RDP follows the Code of Conduct and the Use of Data in Health Research and has been approved by the Biobank Board of the Medical Ethics Committee of the University Medical Centre Utrecht (# 12-387 UNRAVEL Biobank). As a part of UNRAVEL, the use of already existing text files (e.g. clinical notes) is exempt from the Medical Research Involving Human Subjects Act (WMO) as per judgement of the Medical Ethics Committee (“Text-mining in cardiovascular notes”, 18/446, Utrecht, the Netherlands)
Design
Based on in-house clinical protocols, phenotyping of participants includes medical history, family history, physical examination, routine laboratory testing, 12-lead ECG, chest X-ray, cardiac ultrasound, Computed Tomography (CT) Scan and Magnetic Resonance Imaging (MRI) Scans. These tests are performed upon discretion of the managing physician and have multiple time points in the EHR. Contrary to manual registries, all available data is captured. For example, during a visit at the in-patient clinics several ECG’s can be made per day. Since manually entering all data is a meticulous and laborious effort, not all might be entered into manual registries.
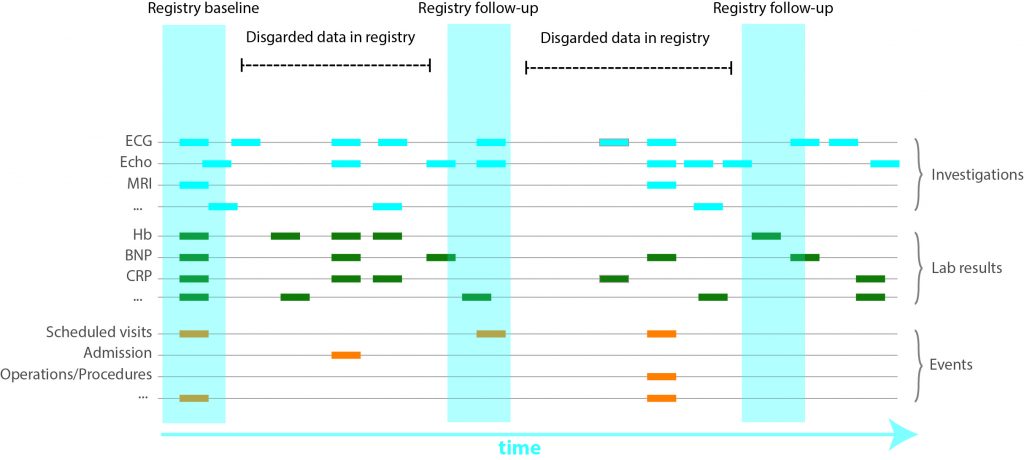
Data is stored in a temporal sequence
Raw data is gathered, processed and standardized for all cardiologic, electrophysiological, imaging and genetic modalities. Weekly, these (numeric) data are automatically extracted to the RDP. Metadata is specific information describing the data (such as date of visitation, type of electrocardiogram (ECG) or managing physician) which have been gathered for logistical and administrative purposes. These meta-data harbour valuable information and are also stored in the RDP. Data are viewed, combined, linked to external databases, and analysed using query-based searches for data-extraction using SAS Enterprise Guide.
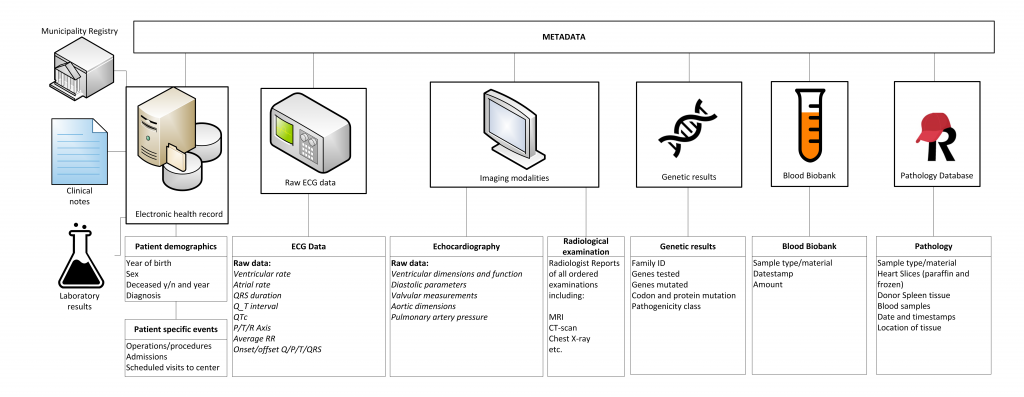
Blood biobank and cardiac tissue database
All patients are asked for collection of biomaterials for the UNRAVEL Blood Biobank. The exact lab protocol is available here.
In short, the standardized biobank protocol consists of one 10 mL serum, one 4.5 mL citrate, one 2 mL, one 10 mL EDTA and one 10 mL Na-heparin blood collection tube. These are processed and aliquoted to two vials of 0.5 mL wholeblood from EDTA tubes, four vials of 0.5 mL plasma from citrate tubes, six mL vials of 0.5 plasma from EDTA and heparin tubes and six vials of 0.5 mL serum All samples are stored at -80° C. Availability, type, and storage of material is linked to the RDP for easy accessibility.